|
Stink-bombs, rotten eggs, garlic and other sulphurous smells, fire and brimstone, sulphuric acid and acid rain all owe their nastiness to sulphur the next element in the table – or even sulfur if you prefer the American spelling. Number 16 in this increasingly periodic series of articles on the elements (it’s months since the last article an years since I started it on a whim back in 2001 thinking I would write an article on each element in an hour or less). Number 16 covers the 16th element in the periodic table. Like Phosphorus, Sulphur is another element associated with the fires of Hell – ‘tis even mentioned in the Bible. Brimstone is sulphur in its native state, i.e. hot and smelly, as if just emerged from a volcano. And, unbelievably, today there are still miners working in and beneath one active volcano, named Kawah Ijen, in Java where they retrieve yellow sulphur for their employer at a princely wage of $2 a day at a time when the minimum wage in Ireland is 7.65 euro – per hour or 50 dollars for an eight hour day. Can you imagine working in hellish conditions, that can only be described as Dantesque, where you are surrounded by belching sulphur-laden steam clouds coming up from deep within the Earth’s active crust?
Sulphur is inextricably linked with bad smells. Put sulphur with hydrogen as hydrogen sulphide, or H2S as the chemists fondly refer to it, and you may conjure up the smell of bad eggs. Or remember that smell from the bad sewer that used to run beneath Castle Street before USSR and USA started their cold war against leaky or overflowing sewerage pipes in Castlebar? Or if you’re a rural type then think about that certain whiff that wafts from your neighbour’s septic tank on occasion? H2S may look quite close to H2O in chemical structure but H2S is responsible for that classic rotten egg smell. You can smell it at very low concentrations. But, strangely, above a certain concentration in the air you can no longer smell sulphur dioxide. And when you reach that stage it becomes extremely dangerous and possible even deadly.
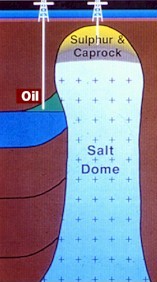
Sulphur is extracted from geological deposits in the Earth's crust.
|
Have you ever mistaken garlic for a gas leak? The similarity between the ‘smell of gas’ and that of garlic emanating from the over as you cook up a roast chicken with a few cloves of garlic inside that oh so tasty rubber chicken from the supermarket freezer is no coincidence. Both are based on the smell of the mercapto group i.e. H-S where H is hydrogen and S is the symbol for sulphur. Garlic’s smell is due to a compound called allyl mercaptan. It’s fascinating but a somewhat similar compound, called butyl mercaptan, is added to gas along with dimethyl sulphide to give that characteristic warning odour of leaking gas. For me, at least, there are some mercapto compounds that are absolutely without challenge the most disgusting, gut-wrenching smells you will ever come across. Hydrogen sulphide is like Chanel Number 5 in comparison with some of the real ultra-nasty mercaptos – so vile are they. I’ve only ever come across these in laboratories but believe me you don’t want some of these sulphur smells wafting around while you are trying to eat breakfast – serious danger of losing said breakfast.
The similarity between hydrogen sulphide and water H-S-H and H-O-H is obvious when you write it down. But in fact this similarity does go much deeper. This is in the sense that certain organisms use hydrogen sulphide in a very similar way to the way that organisms who belong to shall we say ‘mainstream biology’ use water. Not for washing with or swimming in, but for a more basic biochemical function, i.e. driving biochemical reactions that keep the organism going. Sulphur bacteria take over in locations where oxygen is absent – providing energy in the depths of polluted seas such as the Baltic, for example. The bottom of the Baltic has thousands of square kilometres of ocean floor that are now covered in mats of the sulphur bacteria Beggiatoa where once a more aerobic community survived. In this situation they are an indication of lack of oxygen and environmental deterioration.
It’s only recently that Irish farmers have started putting sulphur on their fields again. For years it was supplied free of charge from the atmosphere. But then the campaign against ‘Acid Rain’ started and the big oil companies and coal users were forced to switch to low sulphur fuels. For some reason the Norwegians and Swedes objected to having their fish killed by sulphuric acid from Central Europe falling from the sky and acidifying their lakes. Sulphur is a contaminant of many fossil fuels – and once up the stack of a power station as sulphur dioxide to sulphurous acid and to sulphuric acid in the little droplets surrounding tiny cloud-forming particles. And when it rains down again it’s bye-bye little fishes in all those Norwegian lakes – or anywhere that doesn’t have the capacity to neutralise the acidity of the rain. Most people don’t realise that normal rain is acidic enough in the first place but add that extra sulphuric acid and away you go with something that may be stronger than your stomach acid. Luckily most of Ireland has plenty of limestone to neutralise the incoming acidity and the Atlantic prevailing winds provide a certain amount of protection from our European Neighbours.

German wines tend to have a lot of sulphur added.
|
The death of thousands of lakes in Nordic regions showed quite clearly how we humans can have a massive influence on our environment thousands of miles away from the source. The response to reducing the amount of sulphur being burned in dirty oil and coal is also remarkable and measurable. Our farmers are paying for it now and having to buy special sulphur-added fertiliser to compensate for sulphur deficiencies that are now much more common than they used to be. It underpins too the case for taking action to prevent global warming. No one can say that we humans do not have a worldwide impact on the planet on its soils, water and air. The acid rain story is the perfect illustration of how we impact all three. Now of course sulphur is a by-product from the fossil fuel industry and conferences are held to study potential uses for the surplus of sulphur. No doubt they will think of something.
Of course before the advent of antibiotics sulphur compounds were essential as medicines, fumigators and sterilants from a medical point of view – often the last resort against disease.
Even today there are at least sixteen officially listed sulphur-based preservatives with E-numbers including sulphuric acid itself. I know that I don’t particularly like many German wines because of the amount of sulphur they like to add in the making – giving them that slightly whiffy taste as a result. One of those namby-pamby websites warning you against eating anything with an E-number in it says that sulphur salts will give you headaches, intestinal upsets, skin disorders, and will destroy your vitamin B12 supply into the bargain. So not only is sulphur smelly but it’s gonna kill you as well!
|
E-Number Food Additives Containing Sulphur
|
|
E220
|
Sulphur Dioxide
|
|
E221
|
Sodium Sulphite
|
|
E222
|
Sodium Hydrogen Sulphite
|
|
E223
|
Sodium Metabisulphite
|
|
E224
|
Potassium Metabisulphite
|
|
E226
|
Calcium Sulphite
|
|
E227
|
Calcium Hydrogen Sulphite
|
|
E513
|
Sulphuric acid
|
|
E514
|
Sodium sulphates
|
|
E515
|
Potassium sulphates
|
|
E516
|
Calcium sulphate
|
|
E517
|
Ammonium sulphate
|
|
E520
|
Aluminium sulphate
|
|
E521
|
Aluminium sodium sulphate
|
|
E522
|
Aluminium potassium sulphate
|
|
E523
|
Aluminium ammonium sulphate
|
Previously: Phosphorus a Piddling Little Element
Next: Catastrophic Chlorine
|